Arbocat project
About ArboCat:
Recurrent outbreaks of arboviruses in endemic regions of the world represent a serious threat of potential propagation to naive settings, where population is immunologically naive.
Lack of previous exposure and the ongoing geographical expansion of viable vector populations has fostered the implementation of preventive strategies in those areas more liable to disease importation.
Catalunya receives a wealth of travellers from both Southeast Asia and the Caribbean and around 700 cases of imported arbovirosis (totalling dengue, chikungunya and zika), have been notified in primary care Health centres, traveller advice public Health services and main hospitals. With the large asymptomatic proportion of infections well-known for these diseases, the threat for autochthonous outbreaks increases in those areas that, for particular environmental and socio-demographic conditions, might be more prone. Having precise knowledge what those areas and cities are becomes a matter of priority.
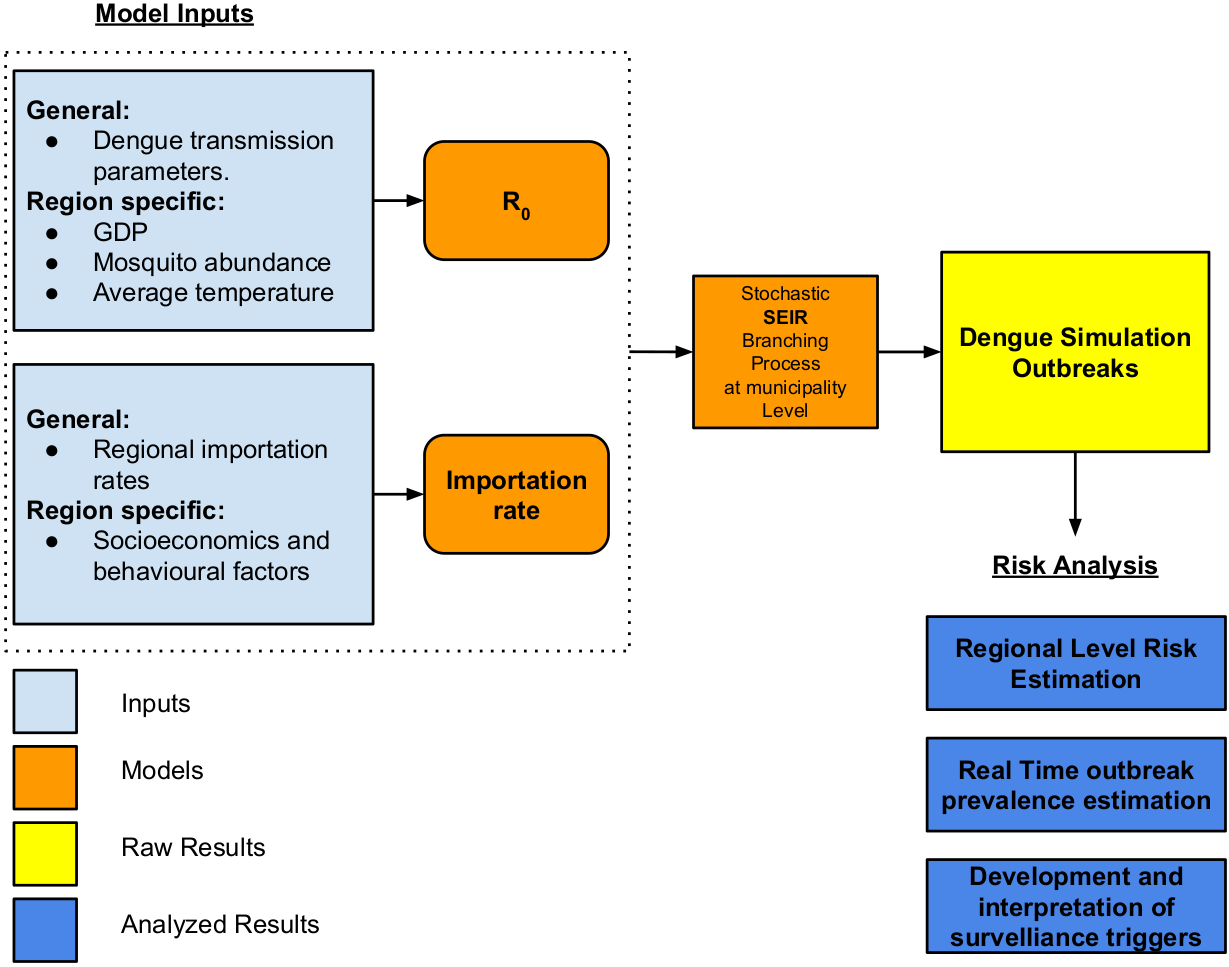
ArboCat is a new platform for the prediction of autochthonous outbreaks of arboviruses implemented for Catalonia with a municipality resolution (948 cities). ArboCat is formed by three sub-models providing estimates of the importation rates, the basic reproduction number (R0) and fitting a stochastic compartmental model that yields the generation time.
ArboCat provides simulations for the risk of autochthonous outbreaks and the epidemic curves for both current and future ISIMIP2a socioeconòmic and climate change scenarios.

Who we are
ArboCat provides simulations for the risk of autochthonous outbreaks and the epidemic curves for both current and future ISIMIP2a socioeconòmic and climate change scenarios.
The importation model make the assumption that outbreaks originates with importation of infectious cases and as a result generates Catalonia-wide cases importation rate and municipality-specific probability of receiving the next importation. In order to calculate such rates this model takes into account historical arbovirus data; sociological predictors such as municipality population size, poverty indices, education indexes, health insurance measurements, among others.
With this information and after identify relevant predictive variables (by methods such as backward selection or AIC) we can generate the simulates rates through a Bayesian–MaxEnt predictive framework.
Maximum entropy methods are very general ways to predict probability distributions given constraints on their moments. This methods can predict relative abundance distributions based on the number of individuals, species and total energy of the system. Also predict community composition along ecological gradients based on traits, species’ distributions based on environmental covariates and associations in food webs.
The basic reproduction number R0, can be defined as the number of cases one case generates on average over the course of its infectious period, in an otherwise uninfected population. This metric is useful because it helps determine whether or not an infectious disease can spread through a population. When R0 < 1 the infection will die out in the long run. But if R0 > 1 the infection will be able to spread in a population.
The R0 model uses the Ross-MacDonald Formulation
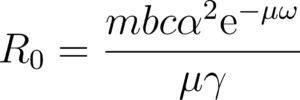
![]() where is the municipality mosquito abundance (modulated by GDP)
where is the municipality mosquito abundance (modulated by GDP)
![]() is the mosquito-to-human prob. of transmission per bite
is the mosquito-to-human prob. of transmission per bite
![]() is the human-to-mosquito prob. of transmission per bite
is the human-to-mosquito prob. of transmission per bite
![]() is the mosquito biting rate
is the mosquito biting rate
![]() is the mosquito (\female) average lifespan
is the mosquito (\female) average lifespan
![]() is the extrinsic incubation period
is the extrinsic incubation period
![]() is the recovery rate
is the recovery rate
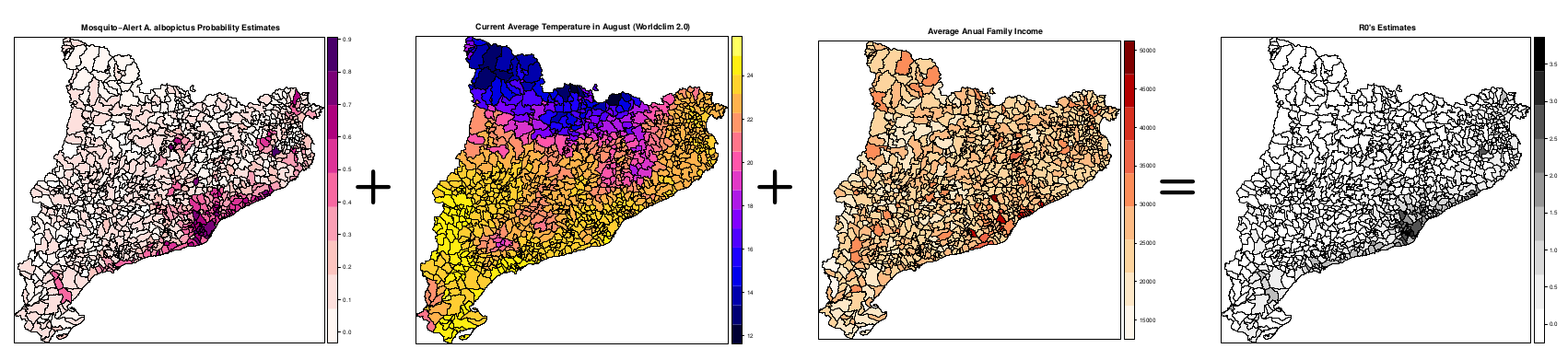
As for now, the data used for this model corresponds to the mosquito abundance provided by Mosquito Alert; average temperatures (worldclim, COSMOS, among others) and the Gross Domestic Product (or some other municipality-level economic factor as a contact modulator). Then R0 computation is made by a Monte Carlo approach in joint with Eq.(1) to produce a parameter distribution for an probabilistic approach
As for now, the data used for this model corresponds to the mosquito abundance provided by Mosquito Alert; average temperatures (worldclim, COSMOS, among others) and the Gross Domestic Product (or some other municipality-level economic factor as a contact modulator). Then R0 computation is made by a Monte Carlo approach in joint with Eq.(1) to produce a parameter distribution for an probabilistic approach
After generating these two parameters (Importation risk and R0) we can simulate the outbreaks using a compartmental like SEIR model.
Compartmental models are a technique used to simplify the mathematical modelling of infectious disease. The population is divided into compartments, with the assumption that every individual in the same compartment has the same characteristics. In the case of SEIR models the population is divided in four compartments: S (for Susceptible); E (for Exposed); I (for Infected) and R (for Recovered). For many important infections (such as arbovirus) there is a significant incubation period during which the individual has been infected but is not yet infectious themselves. During this period the individual is in compartment E.
Arbocat uses a SEIR Markov Branching Process to model the outbreaks. Simulations start with a single imported case. Temporal evolution is governed by daily probabilities of infecteds to progress through E, I, R states. The model considerate in an explicit way the tracking of imported and autochthonous cases, reported and unreported and in a non-explicit way the tracking of mosquitoes dynamics (fitting of generation time for the E-I transition). The next diagram resumes this whole process.
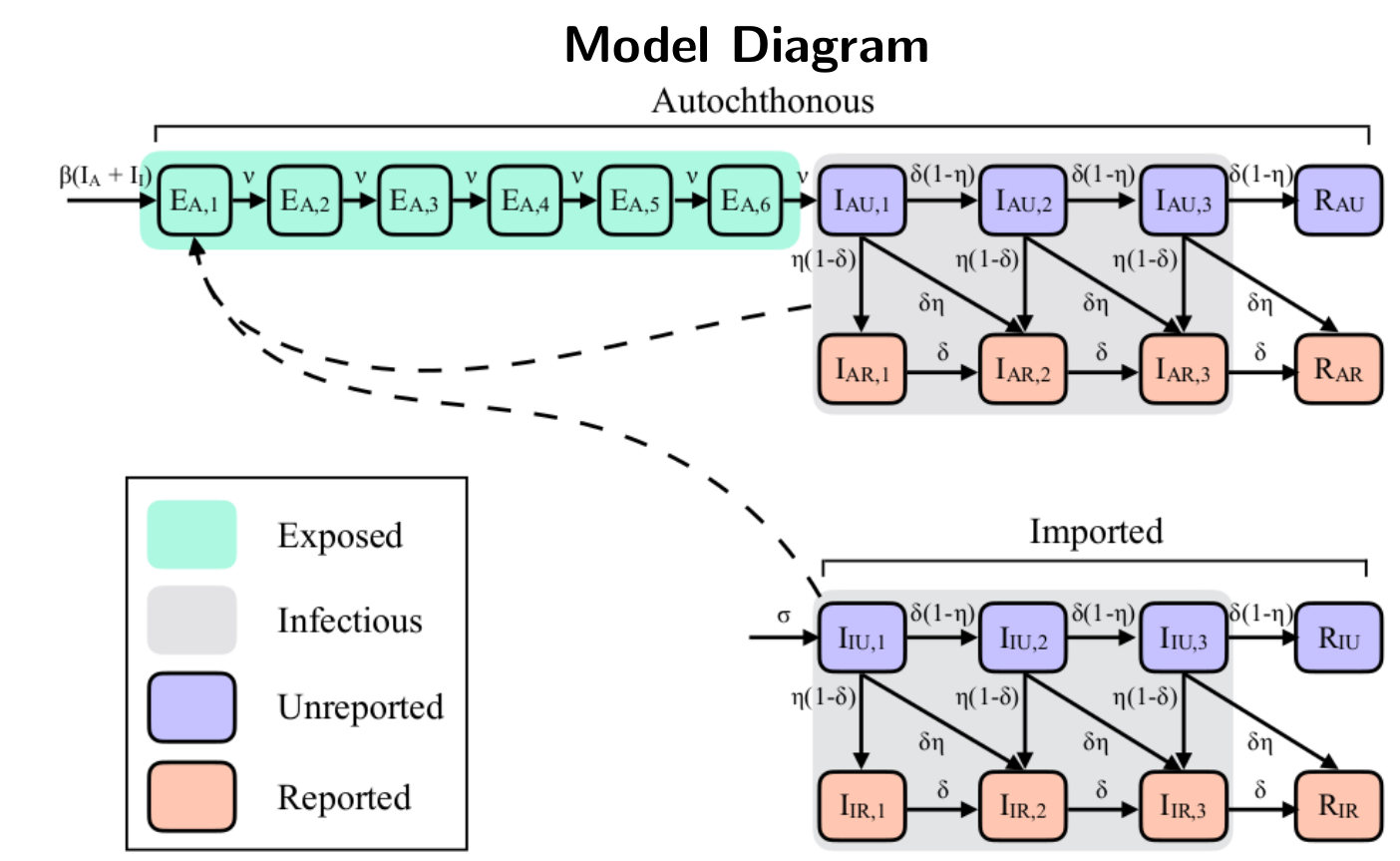
The generation time measures the average duration from initial symptom onset to the subsequent exposure of a secondary case. It corresponds to the sum of the E period and half of the I period. We fit the I period to human DENV viral shedding estimates and then fit the E period so the sum E+I matches the estimated DENV serial interval. In this way the model produce realistic outbreak timings.
ArboCat allows to calculate the Epidemic Expansion Risk using a criteria to classify outbreaks as epidemics (for example more than 10 autochthonous transmissions) and in this way public health experts can design of surveillance triggers through the estimation of probability of epidemic given a number of observed cases in a municipality. Also, by simulating different scenarios for calculating R0 (Climate change, overproliferation of vectors, economic projections and their impact in population’s socio-economic variables) it’s possible to simulate future epidemic scenarios.
ArboCat platform provides a set of tools for the epidemiological analysis of simulated arboviral outbreaks. Following this idea, depending on the initial parameters used to feed the compartmental stochastic SEIR model it is possible simulate different escenarios and get probability distributions and temporal dynamics curves’ for the initial stages of the outbreak.
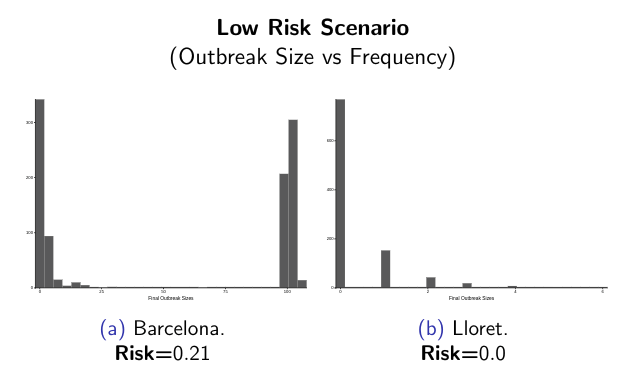
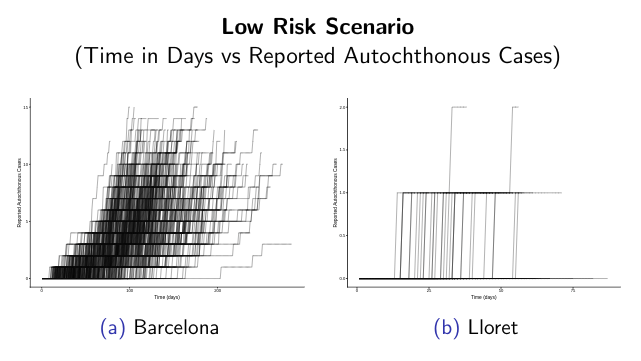
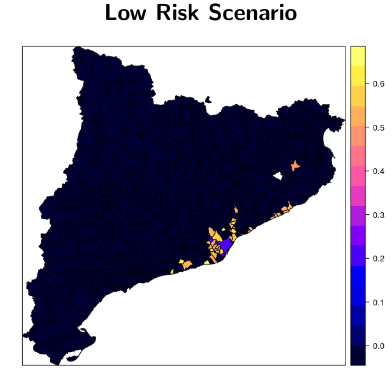
ArboCat is a project under continuous evolution.
Upgrades will be automatically incorporated and uploaded in an attempt to improve the quality of predictions. Our international collaboration with the University of Texas at Austin, as well as our local partners (ASPCat, CREAF, VHIR, PICAT interdisciplinary network) ensure a promising development of future improvements in skill and the capacity of the platform to anticipate public health threats due to arboviral infections in a changing world.







